Electrocardiography (ECG, EKG)
Electrocardiography (ECG, EKG)
In TMC, ECG recordings are performed by two different methods. The first one is to monitor animals during anesthesia with the ECG leads connected to needles inserted subcutaneously in the lower abdomen and lower limbs. The second method is to monitor animals using surgically implanted telemetry devices that animals are conscious.
- Unconscious ECG recordings
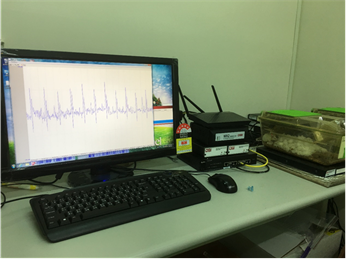
The PowerLab and Lab chart program provide a comprehensive set of tools that identify and analyze various parameters of human or animal ECG recordings. Analysis can be done online or offline and data can be reanalyzed at any time.
The ECG Analysis Module supports data formatting, filtering, visualization, measurement and calculation of electrocardiograph. It is able to detect and report heart rate, PQRST onset, waveform, amplitude, intervals, averaging view, waterfall plot (This displays a 3D plot of averaged beats from one channel. You can decide disease of heart by PQRST waveform.), poincaré plot (The plot shows the variation of each R wave), period histogram (This plot represents the interaction between sympathetic nerve and parasympathetic nerve.), delta NN histogram (Same as period histogram), tachogram (showing RR interval length versus beat number. The tachogram usually gives a good indication of misidentified R waves — peaks that have been falsely identified that appear as sudden dips.)
PowerLab 8/30及Animal Bio Amp is used for this service to obtain

- Conscious ECG recordings- Telemetry Electrocardiography
Electrocardiography (ECG) is recorded in consciously free-moving mice after subcutaneous placement of a DSI transmitter. Briefly, mouse is anesthetized with intraperitoneal injection of Zoletil (10mg/kg, Virbac, Carros, France) and dexdomitor (0.2mg/kg, Pfizer, Espoo, Finland). The mouse is surgically implanted subcutaneously with a DSI radiotelemetric transmitter (model TA11ETA-F10; DSI, St. Paul, MN, USA) at the lower back. The negative and positive leads are placed on the right upper chest and on the left abdomen, respectively. To prevent confounding effects of surgery, we allow the mice to recover for 10 to 14 days after post-surgery. After recovering from surgery, the conscious mouse is placed on a receiver (RPC-1, DSI, St. Paul, USA) for ECG acquisition. After 10~20min habituation, the basal level of ECG waveform is recorded for about 20min followed by 2hr continues recording after drug administration (i.p. or p.o.).
The ECG waveform analysis is performed via telemetry data acquisition system (Data Sciences International) and Ponemah physiology platform software (version 6.12). Basal level of the cardiac function is determined at 10-15 min before drug administration and drug effects are determined at 5 min, 10 min, 15 min, 20 min, 30 min 60 min, 90 min and 120 min after drug administration. Each time point result is 5min average. The changes in heart rate, RR interval and QT interval are determined from ECG.
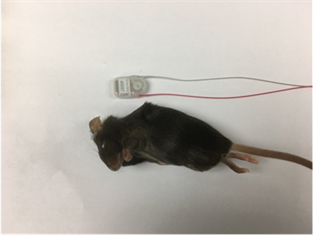
For mouse ECG recording, the DSI radiotelemetric transmitter is implanted subcutaneously while the leads are placed on the right upper chest and on the left abdomen and tacked to the muscle using suture.

For ECG monitoring, the conscious mouse is placed on receiver. The scheduled recordings start 10 to 14 days post-surgery. The ECG waveform analysis is performed via DSI system and Ponemah physiology platform software (version 6.12)
